what is the study about?
Cognitive-behavioural therapy (CBT) is an effective talking treatment for anxiety disorders, but courses are long and difficult to access. However, we have recently shown that even a single session of CBT already has an effect on anxiety. On the other hand, research has shown that a simple blood pressure medication called losartan can improve cognition and memory. In this study, we would like to test whether combining a single-session of CBT with losartan can improve effects on anxiety, and what the underlying brain mechanisms of such an enhancement effect are. We would like to test two groups of people with panic attacks: one who receives CBT after having taken a single capsule of losartan, and one who receives CBT after having taken a placebo capsule.
What is Cognitive-behavioural therapy (CBT)?
CBT is a form of psychotherapy based on the concept that the way we think about things affects how we feel emotionally. Thus, it aims at helping people to modify the way they think and the way they act. The underlying assumption is that learning processes play an important role in overcoming anxiety. This type of therapy is very effective in the treatment of anxiety disorders. Our previous work suggests that the majority of people experience a significant improvement in their symptoms after only one session.
what is LOSARTAN?
Losartan is a medication for high blood pressure which has had UK market authorisation for 20 years. Recent research suggests that this drug can also improve cognition and the effects of learning experiences. As a side effect, some participants experience symptoms such as drowsiness, muscle weakness, low blood sugar, headaches, hypotension or allergic reactions. However, if you are randomised to the losartan condition you will only be asked to take a very low, single dose of 50mg, which is equivalent to what you would be prescribed daily if you had high blood pressure. Recent studies have shown that taking such doses is very unlikely to cause side effects.
what does the study involve?
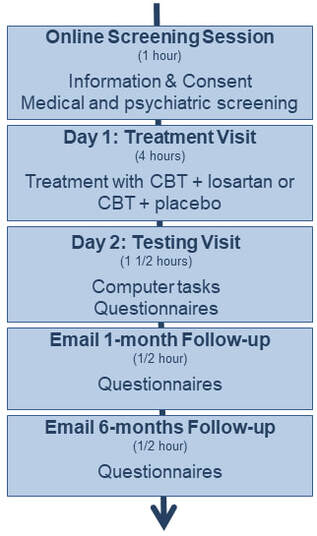
If you decide to volunteer, you will be asked to talk to us online once and come to the Department of Psychiatry, University of Oxford, on two occasions.
1. Online Screening Session
During your first session, we will make sure that it is safe for you to take part, and that you are suitable for this particular study. We will also give you the chance to ask any questions you might have. This session will involve asking you to give us some information about your medical history and to answer some detailed questions about your psychological well-being.
2. Treatment Visit
During this visit, you will receive a session of CBT, either in combination with 50mg losartan or placebo. Which one you will receive will be randomised, and until the end of your study participation, neither you nor the experimenter will be aware which treatment you have received.
3. Testing Visit
On the day following your treatment, we will ask you to work on a few computer tasks. This will take about 1hr and we will ask you to respond via a button box to simple cognitive tasks displayed on a screen. We will also ask you a few questions about your anxiety.
4. Follow-ups
One month and 6 months after your treatment, we will re-assess your anxiety. For this, we email you a set of questionnaires to return to us.
We will be able to pay for overnight accommodation where indicated. We will also cover your travel expenses, and we will reimburse you for your time.
1. Online Screening Session
During your first session, we will make sure that it is safe for you to take part, and that you are suitable for this particular study. We will also give you the chance to ask any questions you might have. This session will involve asking you to give us some information about your medical history and to answer some detailed questions about your psychological well-being.
2. Treatment Visit
During this visit, you will receive a session of CBT, either in combination with 50mg losartan or placebo. Which one you will receive will be randomised, and until the end of your study participation, neither you nor the experimenter will be aware which treatment you have received.
3. Testing Visit
On the day following your treatment, we will ask you to work on a few computer tasks. This will take about 1hr and we will ask you to respond via a button box to simple cognitive tasks displayed on a screen. We will also ask you a few questions about your anxiety.
4. Follow-ups
One month and 6 months after your treatment, we will re-assess your anxiety. For this, we email you a set of questionnaires to return to us.
We will be able to pay for overnight accommodation where indicated. We will also cover your travel expenses, and we will reimburse you for your time.
inclusion and exclusion criteria
We are looking for participants over the age of 18 who are experiencing panic attacks. You may not be able to take part if you:
- have a history of epilepsy or seizures, or other severe diseases such as kidney, liver, heart or respiratory problems
- have a history of a psychotic disorder or bipolar disorder
- are pregnant or breast-feeding
- are taking certain medication
- will not be able to refrain from benzodiazepines for 48 hours before the sessions.
what previous participants say
"I cannot thank you enough for how you have helped me and hope you will continue to go on to help many others who are trapped in this awful condition."
"Just wanted to say that life is still absolutely fantastic after the study. We have just returned on a 12 hour flight and I sailed through it!! Every time I achieve something like this it makes me think of you and the amazing change you have made to my life."
"I am finally beginning to believe it is possible to live a life free of anxiety and panic attacks and that's an amazing thought!"
"The work you do is so amazing and I know you are able to see the short term results; I just wanted you to know that it truly is a gift that keeps on giving."
"Just wanted to say that life is still absolutely fantastic after the study. We have just returned on a 12 hour flight and I sailed through it!! Every time I achieve something like this it makes me think of you and the amazing change you have made to my life."
"I am finally beginning to believe it is possible to live a life free of anxiety and panic attacks and that's an amazing thought!"
"The work you do is so amazing and I know you are able to see the short term results; I just wanted you to know that it truly is a gift that keeps on giving."
study materials
| participant_information_sheet_version_6.pdf | |
| File Size: | 195 kb |
| File Type: | |
| Panic Screening Questionnaire.pdf | |
| File Size: | 98 kb |
| File Type: | |
contact
Please get in touch if you would like to take part in this study, and do not hesitate to also let us know if you have any questions or worries about a potential participation.
Dr. Andrea Reinecke
Group Leader
Email: [email protected]
Phone: +44 01865 618320
Dr. Andrea Reinecke
Group Leader
Email: [email protected]
Phone: +44 01865 618320

